Portfolio
Our portfolio is a result of High Throughput Screening (HTS) of a diverse library containing lead-like and drug-like compounds to discover small molecule inhibitors of accumulation of insoluble glycogen or polyglucosan bodies in Adult Polyglucosan Body Disease (APBD) skin fibroblasts. Taking advantage of the robustness of fibroblasts as a cellular model for APBD and the common pathological feature in Glycogen Storage Disorders (GSDs), we believe that developing pharmaceutical inhibitors of glycogen accumulation is likely to benefit most GSDs. Moreover, as our molecules are likely to significantly affect cellular metabolism and vital cellular pathways, which are strongly connected to many diseases, we aim to integrate research derived data from GSDs in-vivo and in-vitro models and to develop our molecules as therapies for other disorders that are implicated with similar cellular pathologies.
Ex-vivo High-Throughput Screening (HTS)
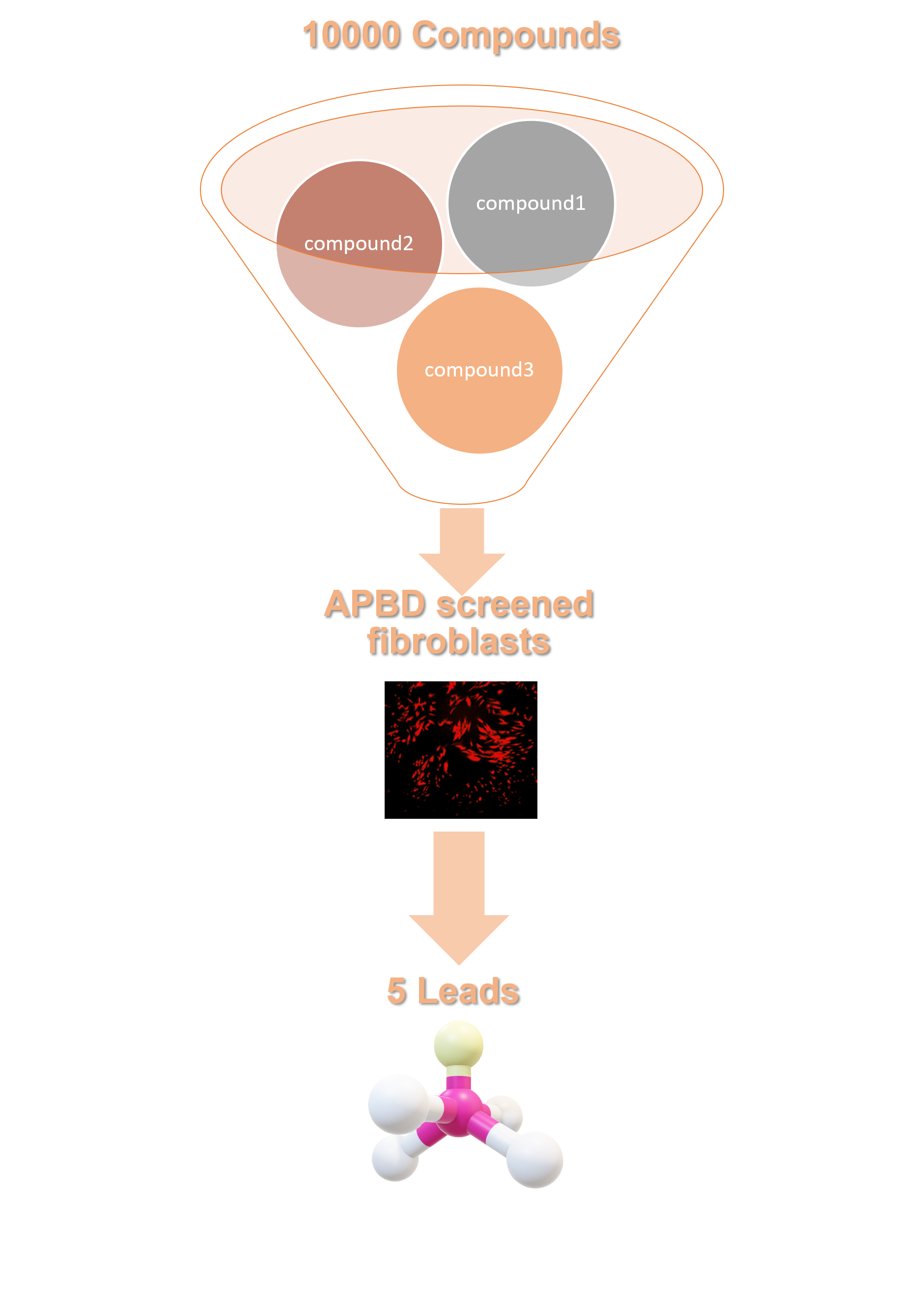
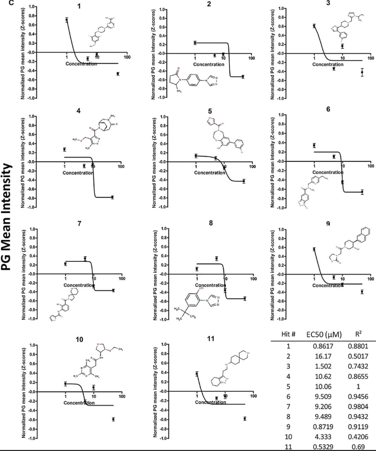
Our novel compounds were discovered utilizing primary cells from APBD patients and the HTS technology developed in Tel Aviv University and Hadassa Medical Center.The unique combination of bio-imaging, computational approaches and structural chemistry enabled us to refine 5 leads that are now further investigated in different in-vitro and in-vivo models. One of our identified hits, GHF-201, has been administered in an urgent compassionate use program in Israel and is now in clinical stage.
HTS pipeline steps
✦ Cell-based assay to detect inhibition of polyglucosan(PG) accumulation in APBD cells with a library of small molecules.
✦ Assay readout: Amylase-resistant fluorescence of Periodic Acid Schiff’s reagent (PAS) staining determining PG levels.
✦ Use of APBD patient derived fibroblasts for screening Software-based analysis of cell images.
✦ Focus on Absorption, Distribution, Metabolism, Excretion, and Toxicity (ADMET) compatible (novel and ‘lead-like’) compounds.
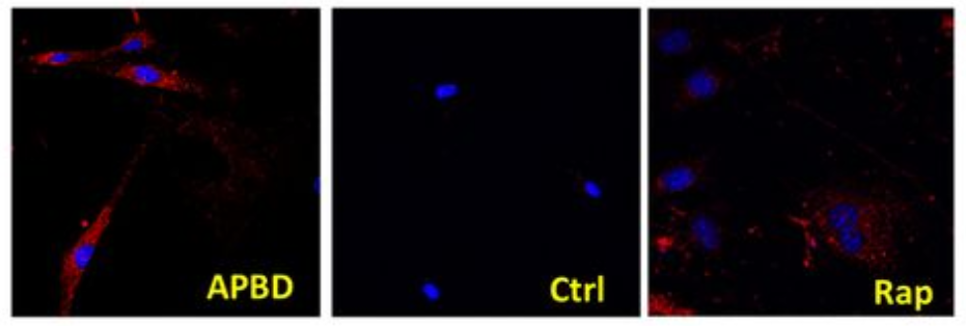
APBD - PG staining in un-treated APBD cells.
Ctrl - PG staining in un-treated Control cells
Rap -PG staining in APBD cells treated with Rapamycin.
(Compound A)
(Guaiacol)
GHF-201
Our leading candidate has been investigated thoroughly in 4 GSDs- Von Gierke, Pompe, Cori, APBD and one LSD– MPS1 and has showed a promising effect in both in-vitro and in-vivo models. in addition, preliminary in vitro studies in several other neurodegenerative disorders showed a potential positive cellular effect by GHF-201.
Mechanism of action
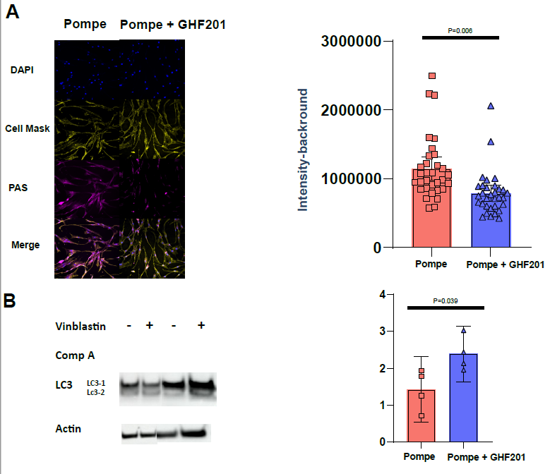
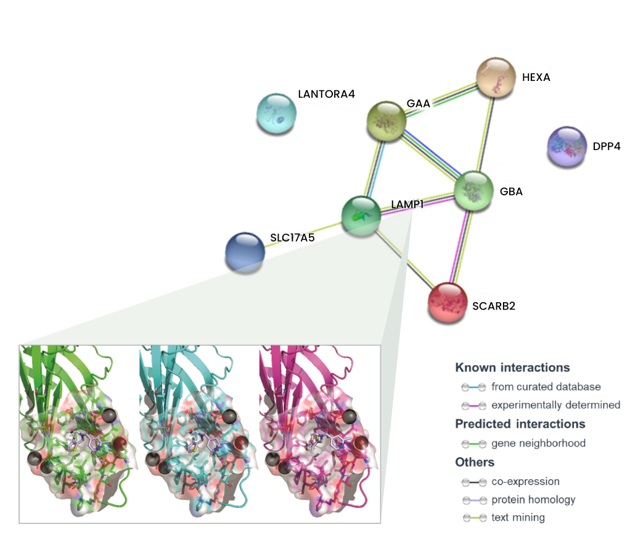
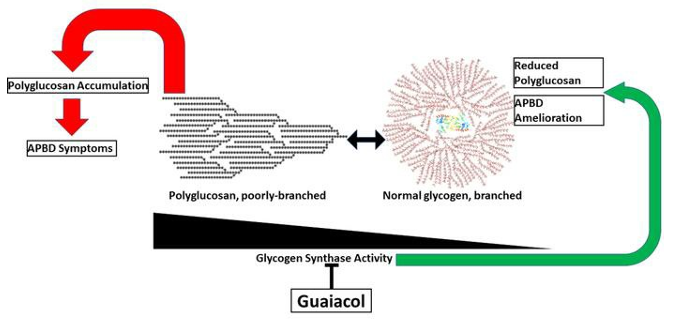
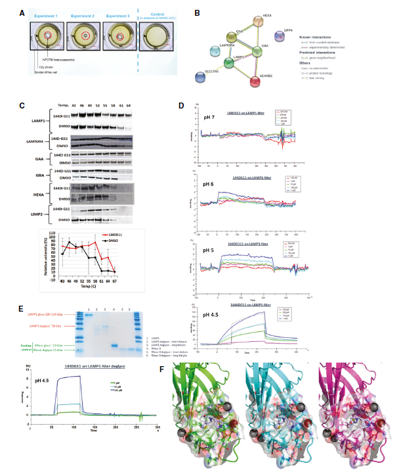
Our extensive in vitro analyses demonstrate that GHF-201 targets Lysosomal-associated-membrane-protein-1 (LAMP1), a scaffold protein on the lysosome membrane essential for the autophagic pathway. This interaction leads to a chain of cellular events leading to enhancement of autophagic flux, increase in mitophagy (the process by which damaged or dysfunctional mitochondria are degraded), glycophagy, elevation in ATP production and energy levels, and decrease in oxidative stress. Overall GHF-201 improves lysosomal activity, enabling better degradation of aggregates and transformation of the excessive glycogen into a useful fuel source for the cells.
Kakhlon et al (2021)
in-vitro effect
GHF-201 was studied in patient derived skin fibroblasts from GSD1a (Von Gierke), GSD2(Pompe), GSD3(CORI) and APBD patients. In all of these cellular models, GHF-201 was found to impact several metabolic cellular pathways. Reducing glycogen buildup, increasing lysosomal activity and autophagy, modifying energetic profiles and impacting lysosomal and mitochondrial key proteins.
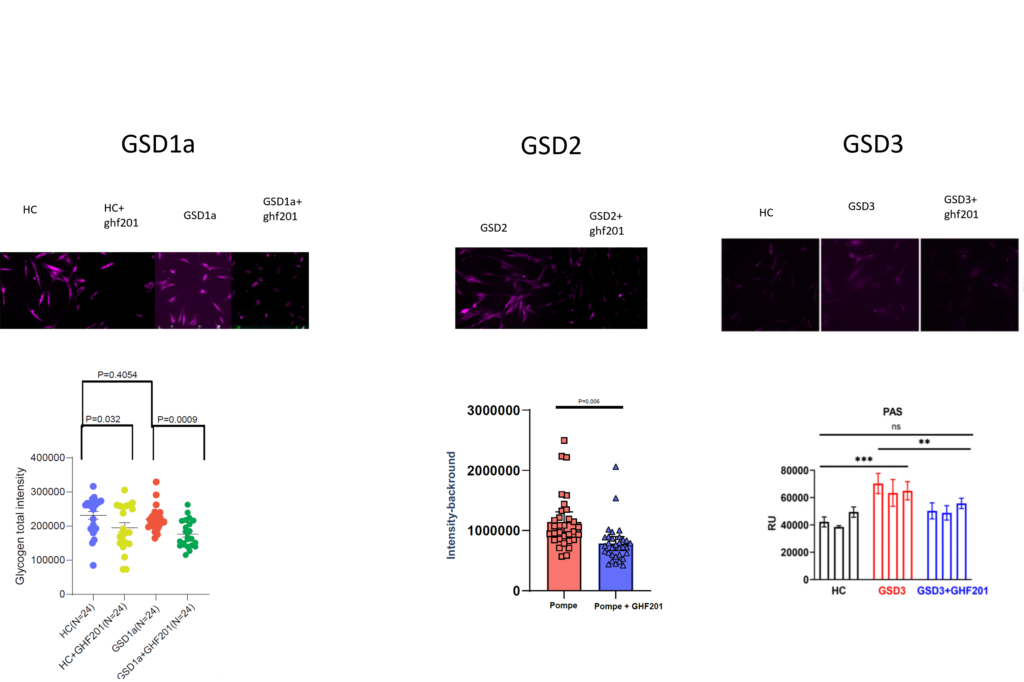
Representative images and quantification of glycogen staining in GSD1a, GSD2 and GSD3 skin fibroblasts treated with GHF201
Sprecher et al (2025)
Sprecher et al (2025)
Mishra et al. (2024)
in-vivo effect
GHF-201 showed consistent disease improvement across four mouse models of GSDs, revealing a powerful effect on cellular metabolism through lysosomal and mitochondrial pathways.
Additionally, in MPS1 mouse model, GHF-201 improved lysosomal activity in several tested organs ,drastically changed the metabolic profile of treated mice and has shown promising effect in motor and behavioral tests.
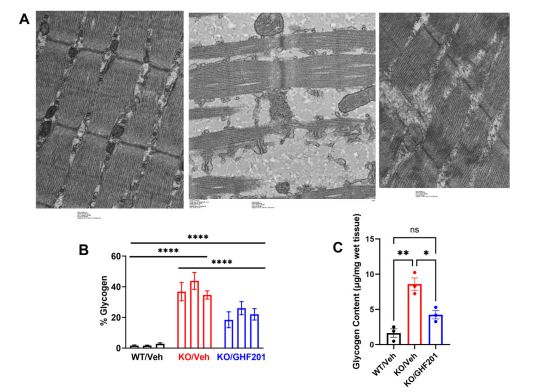
In an APBD mouse model (Gbeys/ys ), GHF-201 was effective in lowering polyglucosan bodies in numerous tissues such as the brain, liver and heart. ATP production was increased in accordance to the carbohydrate catabolism increase caused by GHF-201, and ultimately a notable improvement in survival and motor capacity was observed.
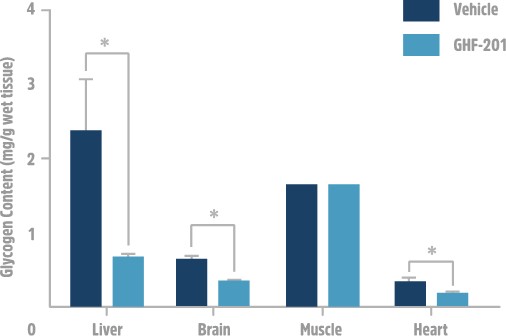
Results from APBD mouse model showing reduction in glycogen from several tested tissues.
Kakhlon et al (2021)
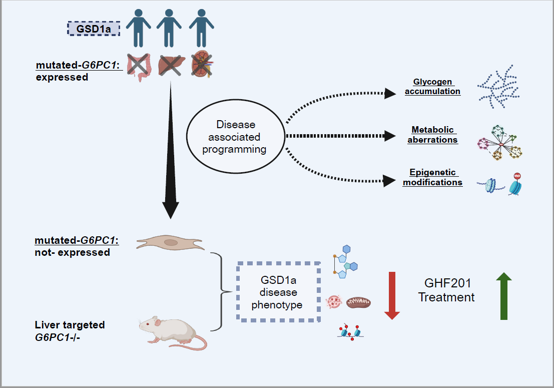
In a GSD1a (Von Gierke disease) inducible mouse model (L. G6pc-/-), GHF-201 drastically improved pathological features by reducing liver G6P and glycogen and increasing liver and serum glucose levels.
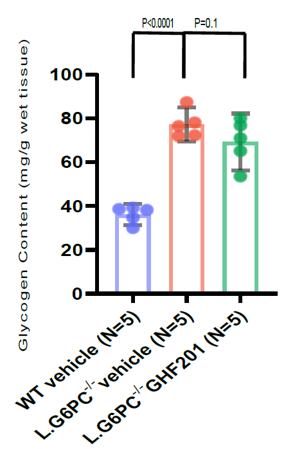
Results from GSD1a mouse model showing reduction in glycogen.
Sprecher et al (2025)
In a GSD2 (Pompe disease) mouse model (Gaa-/-), GHF-201 was demonstrated to be safe and its pharmacokinetic profile revealed biodistribution to brain and muscle. In addition, GHF-201 improved the deficient motor phenotype, the bioenergetic function and autophagic activity of muscle fibers, myotubes and pancreatic islets.
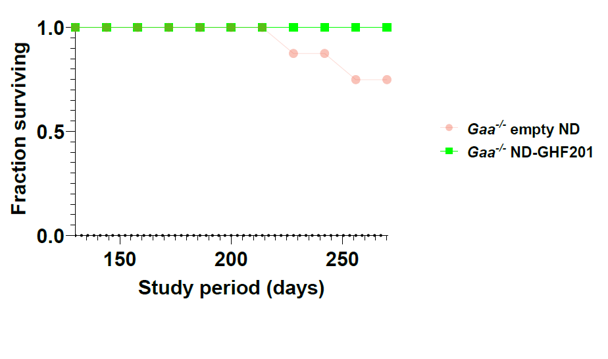
Results from GSD2 mouse model showing improved survival
Sprecher et al (2025)
In a GSD3 (Cori Disease) mouse model (Agl−/−), GHF-201 was able to improve all locomotion parameters and partially reversed hypoglycemia, hyperlipidemia and liver and muscle malfunction. Treated mice burnt carbohydrates more efficiently and showed significant improvement of aberrant ultrastructural muscle features.
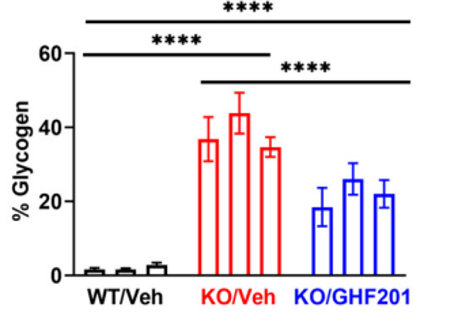
Results from GSD3 mouse model showing reduction in glycogen in muscle
Mishra et al. (2024)
Clinical trials
GHF-201 was approved for an urgent compassionate use program in Israel limited to 3 APBD patients.
GHF partnered with Lyotropic Delivery System (LDS) to formulate GHF-201 in a highly-bioavailable non-aqueous oral solution, to be administered twice daily.
The program, led by Prof. Alexander Lossos and his team at Hadassah University Hospital–Ein Kerem, Jerusalem, Israel, enrolled 3 APBD patients, totaling over 11 patient-years.
GHF-201 did not show any negative safety signals while patients generally reported feeling an increase in their leg strength and energy levels.
Importantly, the pathological metabolite in APBD, polyglucosan, was reduced in skin cells and Lymphocytes of all three APBD treated patients.
The promising clinical improvement of GHF-201 was also indicated by an increase of muscle strength in all three APBD patients and by a reduction in plasma levels of Neurofilament light chain, a known marker for neurodegeneration.
Following a Phase 1 study in 32 healthy volunteers that is nearing completion, a clinical study involving APBD patients is now planned to test GHF-201 for APBD.
The growing knowledge and evidence about GHF-201 effect and mode of action suggest that it may offers a new, safe, and efficacious approach to treat APBD and additional glycogen and lysosomal storage disorders, as well as diseases with greater prevalence.
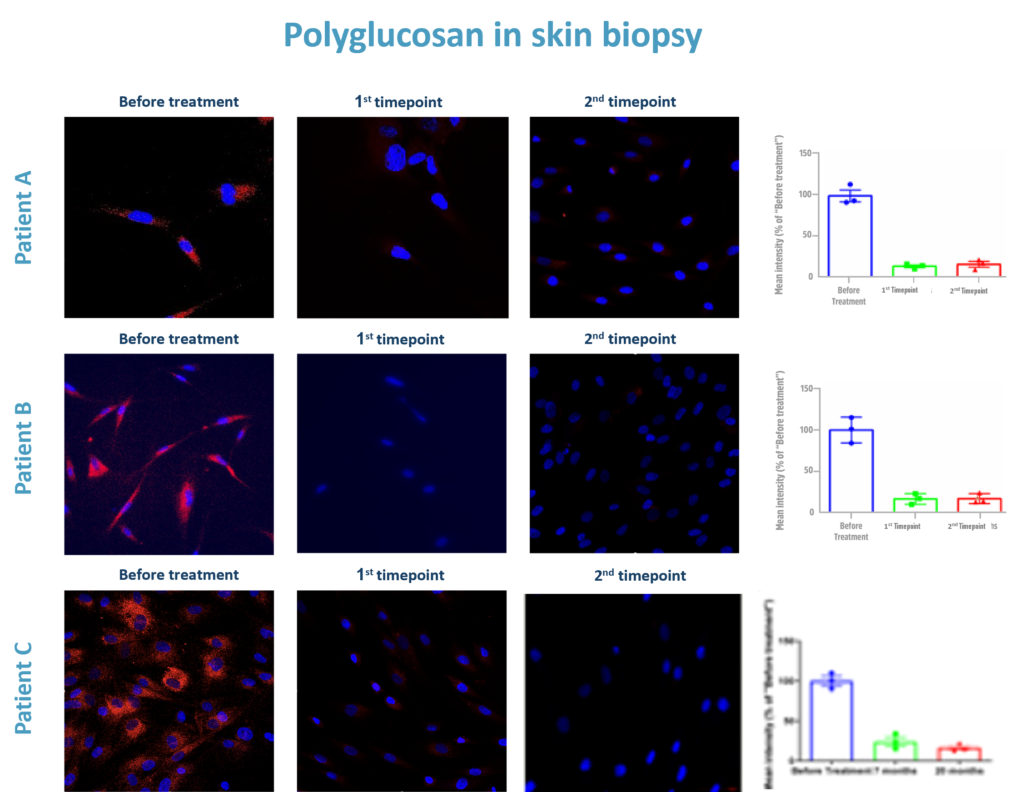
Polyglucosan quantification results from the APBD compassionate trial.
Publications
Human Molecular Genetics, 2015
D. Sean Froese et al.
Journal of Lipid Research, 2017
Rafael Alvarez et al.
Biochemical Journal, 2017
Leonardo J. Solmesky et al.
JCI Insight, 2018
Or Kakhlon et al.
EMBO Molecular Medicine, 2021
Or Kakhlon et al.
biomolecules, 2024
Kumudesh Mishra et al.
bioRxiv, 2025
Uri Sprecher et al.
2025
Uri Sprecher et al.